Cusabio Salmonella typhimurium Recombinant
Abstract
The HIV/AIDS epidemic continues to be a global health problem, especially in sub-Saharan Africa. Therefore, an effective HIV-1 vaccine is urgently needed to mitigate this ever-expanding problem. Since HIV-1 infects its host through the mucosal surface, a vaccine against the virus must elicit both mucosal and systemic immune responses. Oral attenuated recombinant Salmonella vaccines offer this potential to deliver HIV-1 antigens to the mucosal and systemic compartments of the immune system.
To date, a number of preclinical studies have been conducted, in which HIV-1 Gag, a highly conserved viral antigen possessing T- and B-cell epitopes, has been successfully delivered by recombinant Salmonella typhimurium vaccines, and in most cases, HIV-specific immune responses were induced. In this review, the potential use of Salmonella enterica serovar Typhimurium as a live vaccine vector for HIV-1 Gag is explored.
Keywords: Salmonella, vaccine, vector, HIV-1 Gag, immune response
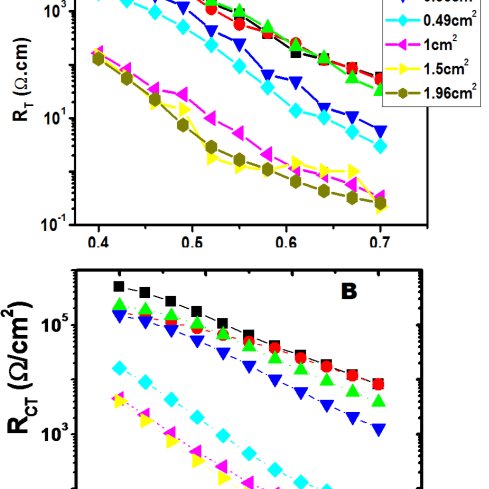
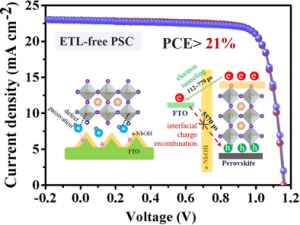
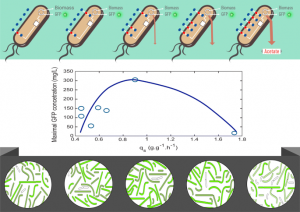
Purity: >85% (SDS-PAGE)
Target Names: cheY
Uniprot No.: P0A2D5
Alternative names: cheY; STM1916; CheY chemotaxis protein
Species: Salmonella typhimurium (strain LT2 / SGSC1412 / ATCC 700720)
Expression Region: 2-129
Protein length: Total length of the mature protein
Label information
The following labels are available.
- N-terminus His-tagged
- Without tags
- The type of label will be determined during the production process. If you have specified a tag type, let us know and we will develop the specified tag preferentially.
Form: Lyophilized powder
Buffer before lyophilization: Tris/PBS based buffer, 6% trehalose, pH 8.0
Reconstitution
We recommend that this vial be briefly centrifuged before opening to bring the contents to the bottom. Reconstitute protein in sterile deionized water at a concentration of 0.1-1.0 mg/mL. We recommend adding 5-50% glycerol (final concentration) and an aliquot for long-term storage at -20℃/-80℃. Our final default glycerol concentration is 50%. Customers could use it for reference.
Storage Conditions
Store at -20°C/-80°C upon receipt, need to be aliquoted for multiple uses. Avoid repeated cycles of freezing and thawing.
Shelf life
Shelf life is related to many factors, storage condition, buffer ingredients, storage temperature and the stability of the protein itself. Generally, the shelf life of the liquid form is 6 months at -20°C/-80°C. The shelf life of the lyophilized form is 12 months at -20°C/-80°C.
Delivery time
The delivery time may differ depending on the way or location of purchase, consult your local distributors for the specific delivery time.
Note: All of our proteins are shipped with regular blue ice packs by default. If you request shipping with dry ice, please contact us in advance and additional fees will be charged.
Notes: Repeated freezing and thawing is not recommended. Store working aliquots at 4°C for up to one week.